Platform
Conditionally Active Biologics™ (CAB)
BioAtla©'s revolutionary patented platform for developing conditionally active therapeutics yields antibodies (as well as other biologics) that can be activated or inactivated under defined physiological conditions.
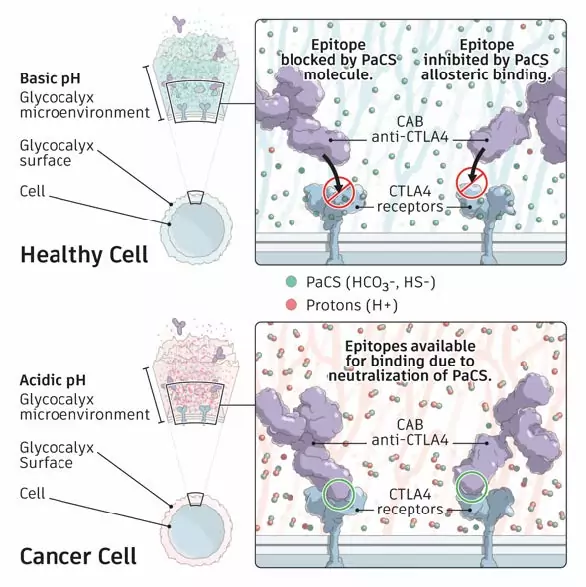
CAB technology takes advantage of the unique microenvironment associated with different diseases and tissues, which can result for example from differences in metabolism between diseased and normal tissue. In cancer the unique cell metabolism described by Warburg contributes to a characteristic microenvironment that activates our antibodies when they are in close proximity to a tumor, and reversibly inactivates our antibodies if they drift away from the tumor.
BioAtla scientists have discovered a novel chemical switch mechanism involving physiological-occurring chemicals, such as bicarbonate and hydrogen sulfide. These molecules are negatively charged at physiological conditions and interact with positive charged areas on the protein surface. Under acidic conditions of the tumor microenvironment they are neutralized and released from the protein surface, uniquely allowing CAB antibodies to bind to their target and attack the tumor cell. BioAtla refers to this novel physiological mechanism, used for generating CABs, as Protein-associated Chemical Switch(es) or PaCS Mechanism.
Features
- The unique physiology and metabolism of a diseased tissue is used to locally activate a biologic, even if its target is also found in normal tissue
- Activation of the biologic is reversible, such that it can be inactivated and reactivated as it passes from diseased to normal to diseased tissue
- No exogenous (for example non-antibody) sequence is introduced
- Can be applied to antibody therapeutics including naked antibodies and ADC's, cell therapies such as CAR-T, enzyme therapies, or other biologics
Benefits
4th Generation Antibody Therapeutics
Strong IP Protection
- 771 patents (499 granted / 7 allowed) patents and 265 pending patent applications cover BioAtla's CAB technology and products
- Worldwide patent coverage with issued and pending applications in all major market/manufacturing countries
-
Broad patent coverage of including:
- Methods of making and screening CAB antibodies
- Different formats (e.g. ADC, multi-&-bi-specific, CAR-T, mAbs and proteins
- Specific antibodies (composition of matter)
- Broad coverage provides a patent minefield where the product is not dependent on a single patent for protection

