BIOATLA REPORTS SECOND QUARTER 2022 FINANCIAL RESULTS AND HIGHLIGHTS RECENT PROGRESS
– Mecbotamab vedotin (BA3011) Phase 2 preliminary observations in Non-Small Cell Lung Carcinoma (NSCLC) supports advancing to the registrational stage of the study; anticipate full interim data set in 4Q22
– Mecbotamab vedotin (BA3011) Undifferentiated Pleomorphic Sarcoma (UPS) and osteosarcoma Phase 2 part 2 enrollment anticipated to begin 4Q22 following requested written feedback from FDA; continuing to enroll additional cohorts
– Mecbotamab vedotin (BA3011) sarcoma Phase 2 top-line interim data support advancing with liposarcoma and synovial sarcoma; next steps under evaluation
– Ozuriftamab vedotin (BA3021) NSCLC and Squamous Cell Carcinoma of the Head and Neck (SCCHN) Phase 2 studies ongoing as planned; NSCLC preliminary cohort interim update on track for 2H22, SCCHN trial ongoing with first patient dosed now anticipated in 3Q22; melanoma enrollment ongoing with status update using new validated Circulating Tumor Cell (CTC) assay anticipated Q422
– CAB-CTLA-4 (BA3071) Phase 1 study ongoing with first patient dosed
– Cash balance of $202.3 million at quarter-end expected to provide funding into 2H24
– Management to host conference call and webcast today at 4:30 PM Eastern Time
SAN DIEGO, August 9, 2022 – BioAtla, Inc. (Nasdaq: BCAB), a global clinical-stage biotechnology company focused on the development of Conditionally Active Biologic (CAB) antibody therapeutics for the treatment of solid tumors, today announced its financial results for the second quarter ended June 30, 2022, and provided an interim topline data update from the mecbotamab vedotin (BA3011) Phase 2 study in NSCLC as well as an operational update on its ongoing clinical programs, including BA3011, ozuriftamab vedotin (BA3021) and CAB-CTLA-4 (BA3071) addressing multiple tumor types.
“BioAtla continues to progress our CAB-ADC programs, most recently with important signals observed this quarter in our Phase 2 BA3011 NSCLC study. In parallel, we continue to advance with our Phase 2 studies for BA3011 in multiple sarcoma subtypes, with positive signals identified in liposarcoma and synovial sarcoma and official enrollments into part 2 for UPS and osteosarcoma expected to begin this year. We also are progressing with our additional clinical assets, ozuriftamab vedotin (BA3021) in Phase 2 NSCLC, melanoma and head & neck cancer, as well as with our Phase 1/2 naked immuno-oncology antibody CAB-CTLA-4 (BA3071) across multiple tumor types, and anticipate several updates later this year,” said Jay M. Short, Ph.D., Chairman, Chief Executive Officer and co-founder of BioAtla, Inc.
“We are very encouraged by our Phase 2 NSCLC preliminary observations, which are consistent with results from our Phase 1 NSCLC and Phase 2 sarcoma studies. Collectively, these data further strengthen the potential opportunity of BA3011 as a best-in-class therapeutic across multiple solid tumor types,” said Scott Smith, President of BioAtla. He continued, “We are also very excited with the continued execution across all the other important programs in our pipeline, including BA3021, BA3071, and BA3182. BioAtla
has a strong cash position to support our robust clinical and preclinical programs into the second half of 2024, and we will remain acutely focused on prioritizing the indications we view have the highest probability of success to maximize value for patients and our shareholders.”
Key Developments, Operational Updates and Upcoming Milestones • Phase 2 Trial of Mecbotamab Vedotin (BA3011, NCT03425279) in Patients with: o AXL-positive NSCLC ▪ Trial ongoing in patients who have previously experienced failure of PD-1/L1, EGFR, or ALK inhibitor therapy (average failure 2.5 lines of therapy; 2 prior lines for non-squamous, 4 prior lines for squamous) ▪ 15 patients enrolled to date, with 9 efficacy-evaluable patients • 4 patients currently on treatment did not yet have the opportunity to be followed for 3 months and 2 patients were not deemed efficacy-evaluable. Of the 9 evaluable patients (7 in the non-squamous adenocarcinoma group and 2 in the squamous cell carcinoma group), a total of 1 complete response (CR) and 2 partial responses (PRs) were observed for a combined objective response rate (ORR) of 33% o In the non-squamous group, 4 of 7 had monotherapy and 3 of 7 had combination therapy o All CR / PRs observed were in the non-squamous group, representing an ORR of 43%, or 3 out of 7 patients (2 PRs were in monotherapy, ORR 50%; and 1 CR in combination therapy, ORR 33%)
• The observations in non-squamous cohort are consistent with the signal observed in the Phase 1 study
• Initiating preparations for discussions with the FDA about part 2 of the registrational study in AXL-positive NSCLC patients
▪ No clinical responses (ORR, PR) observed to date in the squamous cohort in either monotherapy (n=1) or combination therapy (n=1)
▪ BA3011 is generally safe and well-tolerated in both monotherapy and combination with nivolumab in advanced NSCLC patients with no new safety signals observed
▪ Full interim data set of approximately 20 patients anticipated this year o AXL-positive Soft Tissue and Primary Bone Sarcomas
▪ Interim Phase 2 analysis after a minimum of 3 months demonstrates antitumor activity following BA3011 treatment in:
• UPS and osteosarcoma; advancing as separate cohorts into part 2 of the Phase 2 study; additional detail for part 2 will become available following FDA written feedback
• Liposarcoma; PFS rate 60% (n=6); next steps under evaluation
• Synovial sarcoma; PFS rate 50% (n=5); next steps under evaluation
▪ Continuing to enroll across cohorts, awaiting data and will provide an update when available
• Phase 2 Trial of Ozuriftamab Vedotin (BA3021, NCT03504488) in Patients with:
o ROR2-positive NSCLC
▪ Trial enrolling in patients who have previously experienced failure of PD-1/L1, EGFR or ALK inhibitor therapy
▪ Interim update on track for second half of 2022
o ROR2-positive Melanoma
▪ Trial ongoing in patients who have previously experienced failure of PD-1 therapy ▪ Completed successful validation of non-invasive liquid biopsy; implementation as part of study protocol is underway
▪ Enrollment ongoing and actively dosing; anticipate trial enrollment status update in Q422
o ROR2-positive SCCHN
▪ Trial ongoing in patients who have previously experienced failure of PD-1 therapy alone or in combination with platinum therapy
▪ Anticipate first patient dosed in third quarter of this year
• Phase 1/2 Dose-Escalation Trial of CAB-CTLA-4 (BA3071) Across Multiple Solid Tumor Types
o Trial ongoing with first patient dosed and actively screening additional patients across multiple centers with advanced solid tumors
• On track for IND filing for CAB EpCAM x CAB-CD3 bispecific antibody (BA3182) in 4Q22
• Anticipate potential IND filings for a pre-clinical next generation CAB-ADC candidate and a second CAB bispecific in 2023
Second Quarter Financial Results
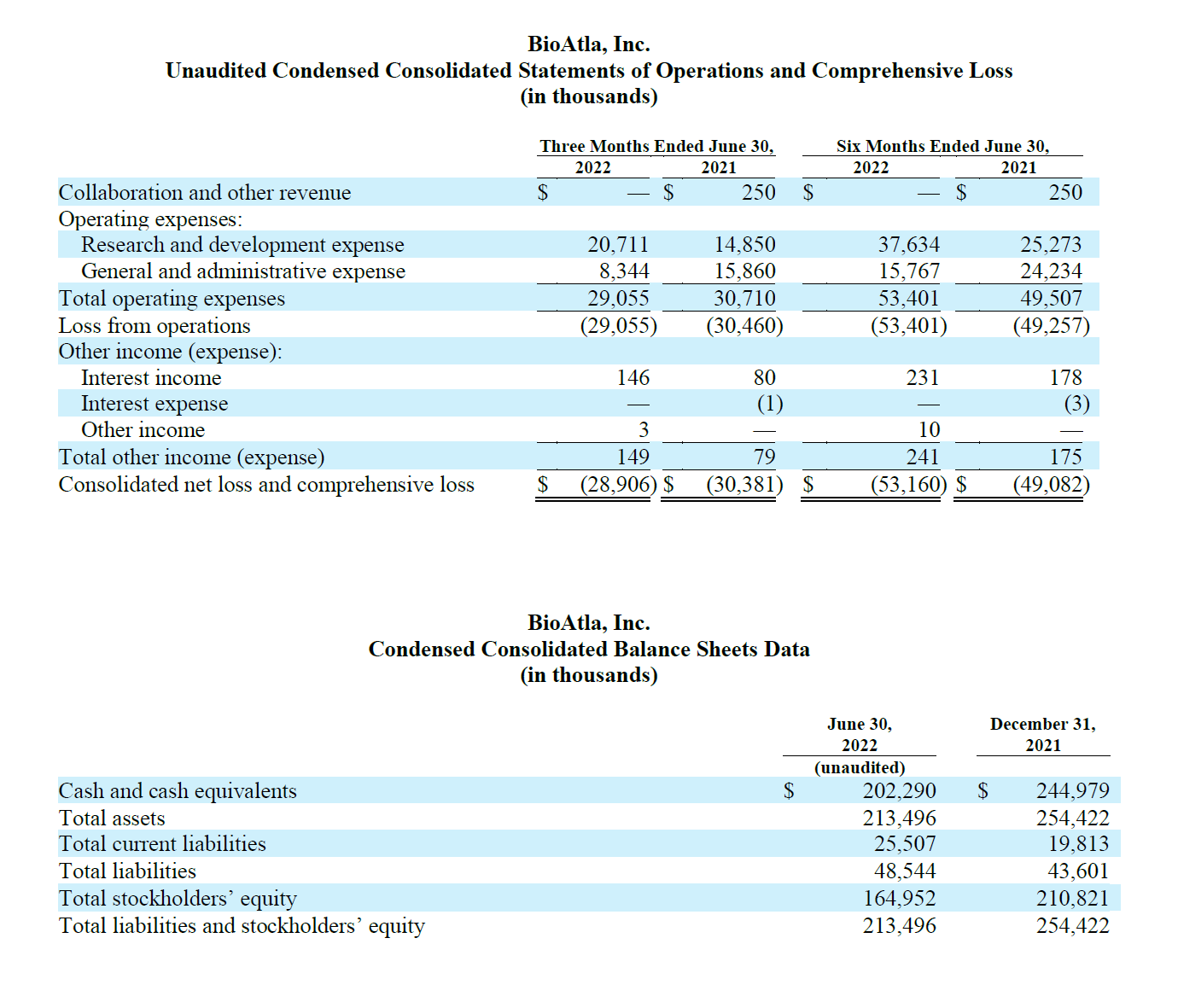
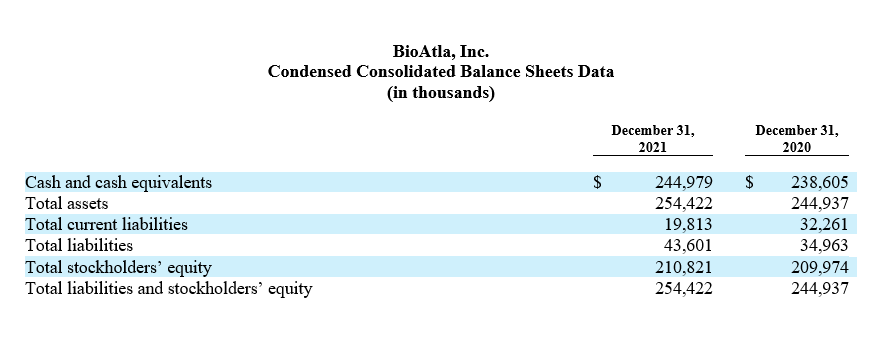
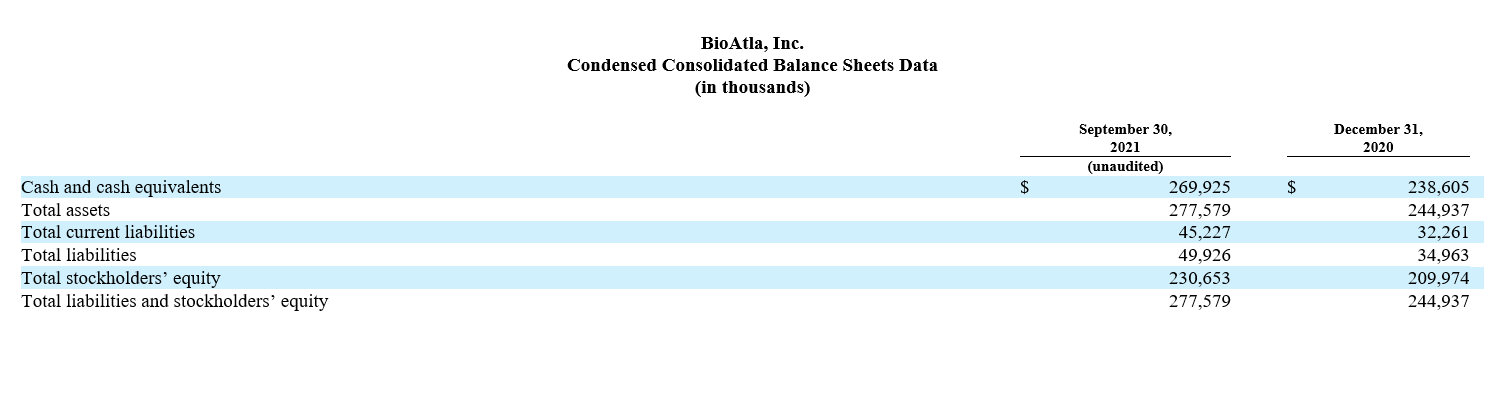
Cash and cash equivalents as of June 30, 2022 were $202.3 million, compared to $245.0 million as of December 31, 2021. We expect current cash and cash equivalents will be sufficient to fund planned operations including all ongoing CAB product development programs into second half 2024.
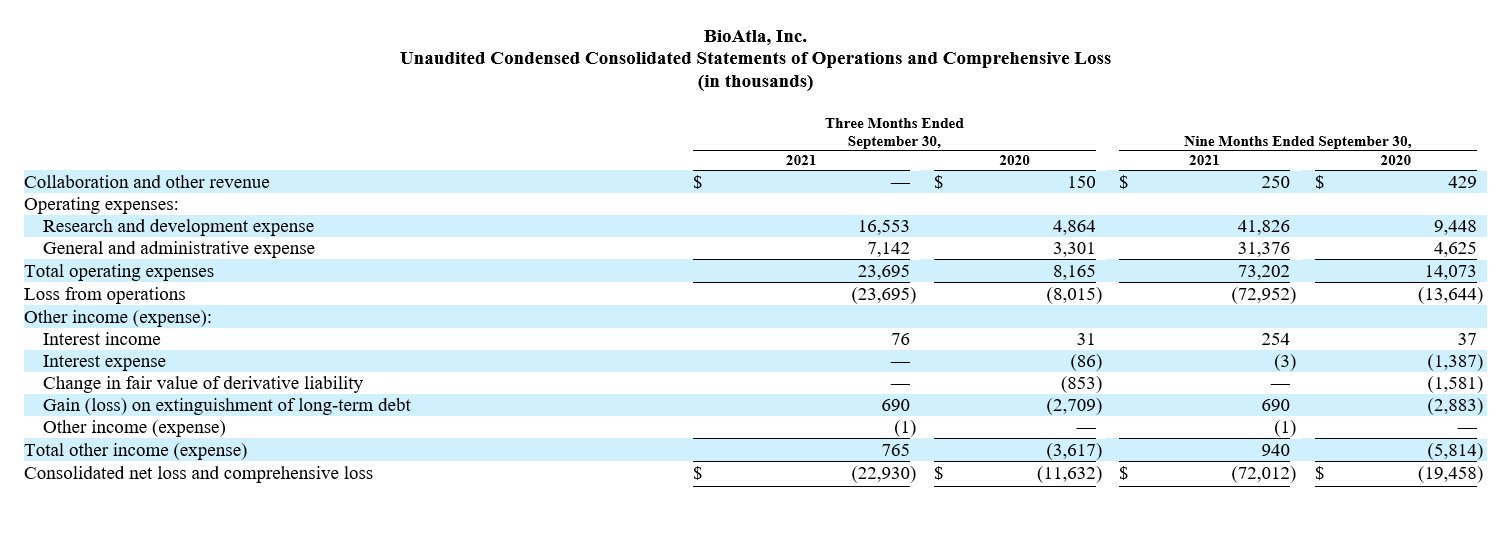
Research and development (R&D) expenses were $20.7 million for the quarter ended June 30, 2022 compared to $14.9 million for the same quarter in 2021. The increase of $5.8 million was primarily driven by expansion of our product development efforts including clinical development for CAB-CTLA-4 (BA3071) and pre-clinical development of additional CAB candidates. We expect our R&D expenses to remain variable from quarter to quarter and generally increase as we continue to invest in R&D activities to advance our product candidates and our clinical programs. General and administrative (G&A) expenses were $8.3 million for the quarter ended June 30, 2022 compared to $15.9 million for the same quarter in 2021. The $7.6 million change was attributle to a decrease in stock-based compensation for the 2022 period. We expect our G&A expenses to moderately increase to support development of our product candidates, advance our intellectual property portfolio, support focused pre-commercialization activities for our product candidate mecbotamab vedotin (BA3011) and satisfy requirements as a public company. Net loss for the quarter ended June 30, 2022 was $28.9 million compared to a net loss of $30.4 million for the same quarter in 2021.
Net cash used in operating activities for the six months ended June 30, 2022 was $42.1 million compared to net cash used in operating activities of $28.5 million for the same period in 2021. The increase in net cash used in operating activities for the first six months of 2022 is primarily due to an increase in research and development expense related to our program development efforts as compared to the first six months of 2021.
Second Quarter 2022 Conference Call and Webcast Details The management of BioAtla, Inc. will host a conference call and webcast for the investment community today, August 9, 2022, at 4:30 pm Eastern Time. A live webcast may be accessed here: https://edge.media-server.com/mmc/p/czrtgcet. The conference call can be accessed by dialing toll-free (844) 826-3035. The passcode for the conference call is 10169204.
A replay of the webcast and slides with topline interim clinical data referenced on the call will be available through “Events & Presentations” in the Investors section of the company’s website after the conclusion of the presentation and will be archived on the BioAtla website for one year.
About Mecbotamab Vedotin (BA3011)
Mecbotamab vedotin (BA3011), CAB-AXL-ADC, is a conditionally and reversibly active antibody drug conjugate targeting the receptor tyrosine kinase AXL. This Phase 2 stage clinical asset is targeting multiple solid tumor types, including soft tissue and bone sarcoma, non-small cell lung cancer (NSCLC) that have previously progressed on PD-1/L1, EGFR or ALK inhibitor therapies. We are also supporting a multi-center investigator-initiated clinical trial in combination with a PD-1 inhibitor in patients with platinum-resistant ovarian cancer, with other potential indications in the future. The Office of Orphan Drug Products (OODP) at FDA granted Orphan Drug Designation to mecbotamab vedotin for the treatment of soft tissue sarcoma. In the Phase 1 clinical study in sarcoma patients mecbotamab vedotin was generally well-tolerated, few patients discontinued due to an adverse event, and no clinically meaningful on-target toxicity to healthy AXL-expressing tissue was observed. Of the seven sarcoma patients who had an AXL tumor membrane percent score (TmPS) of greater than or equal to 70, four of these obtained a confirmed partial response, including patients with leiomyosarcoma, UPS, and Ewing sarcoma. Multiple subtypes of sarcoma present a significant unmet medical need. For example, UPS is one of the most aggressive sarcoma subtypes with the highest recurrence rate and has approximately 4,000 new cases annually in the U.S. There is no FDA approved treatment for UPS and current first and second line therapies are typically limited to doxorubicin, gemcitabine and docetaxel.
About Ozuriftamab Vedotin (BA3021)
Ozuriftamab vedotin, CAB-ROR2-ADC, is a conditionally and reversibly active antibody drug conjugate directed against ROR2, a receptor tyrosine kinase that is overexpressed across many different solid tumors including lung, head and neck, melanoma and breast. We are advancing this Phase 2 stage clinical asset for multiple solid tumor types, including NSCLC that have previously progressed on PD-1/L1, EGFR or ALK inhibitor therapies, melanoma, SCCHN that have previously progressed on a PD-1 inhibitor and ovarian cancers that have previously progressed on platinum therapy.
About BA3071
BA3071, is a CAB anti-CTLA-4 antibody that is being developed as an immuno-oncology agent with the goal of delivering efficacy comparable to the approved anti-CTLA-4 antibody, ipilimumab, but with lower toxicities due to the CAB's tumor microenvironment-restricted activity. This may enable safer anti-CTLA-
4 antibody combination therapies, such as with anti-PD-1 antibody checkpoint inhibitors, and potentially broaden the patient population tolerant to combination therapy and deliver greater efficacy. Like mecbotamab vedotin, ozuriftimab vedotin and our other CAB candidates, BA3071 is designed to be conditionally and reversibly active in the tumor microenvironment. BA3071 is being developed as a potential therapeutic for multiple solid tumor indications, including renal cell carcinoma, NSCLC, small cell lung cancer, hepatocellular carcinoma, melanoma, bladder cancer, gastric cancer and cervical cancer.
About BioAtla®, Inc.
BioAtla is a global clinical-stage biotechnology company with operations in San Diego, California, and in Beijing, China through our contractual relationship with BioDuro-Sundia, a provider of preclinical development services. Utilizing its proprietary Conditionally Active Biologics (CAB) technology, BioAtla develops novel, reversibly active monoclonal antibody and other protein therapeutic product candidates. CAB product candidates are designed to have more selective targeting, greater efficacy with lower toxicity, and more cost-efficient and predictable manufacturing than traditional antibodies. BioAtla has extensive and worldwide patent coverage for its CAB technology and products with more than 600 patents, more than 350 of which are issued. Broad patent coverage in all major markets include methods of making, screening and manufacturing CAB product candidates in a wide range of formats and composition of matter coverage for specific products. BioAtla has two first-in-class CAB programs currently in Phase 2 clinical testing in the United States, mecbotamab vedotin, BA3011, a novel conditionally active AXL-targeted antibody-drug conjugate (CAB-AXL-ADC), and ozuriftamab vedotin, BA3021, a novel conditionally active ROR2-targeted antibody-drug conjugate (CAB-ROR2-ADC). The Phase 1 stage CAB-CTLA-4 antibody, BA3071, is a novel CTLA-4 inhibitor designed to reduce systemic toxicity and potentially enable safer combination therapies with checkpoint inhibitors such as anti-PD-1 antibody. To learn more about BioAtla, Inc. visit www.bioatla.com.
Forward-looking statements
Statements in this press release contain "forward-looking statements" that are subject to substantial risks and uncertainties. Forward-looking statements contained in this press release may be identified by the use of words such as "anticipate," "expect," "believe," "will," "may," "should," "estimate," "project," "outlook," "forecast" or other similar words. Examples of forward-looking statements include, among others, statements we make regarding our business plans and prospects, including potential selective licensing and collaborations, expectations about the sufficiency of our cash and cash equivalents, expected R&D and G&A expenses, the timing and expectations with respect to enrollment in our clinical trials, the timing and success of our clinical trials and related data, plans to advance development of several bispecific CAB candidates, including the timing of potential IND submissions and our market opportunity. Forward-looking statements are based on BioAtla's current expectations and are subject to inherent uncertainties, risks and assumptions, many of which are beyond our control, difficult to predict and could cause actual results to differ materially from what we expect. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. Factors that could cause actual results to differ include, among others: potential delays in clinical and pre-clinical trials due to the global COVID-19 pandemic; other potential adverse impacts due to the global COVID-19 pandemic such as delays in regulatory review, manufacturing and supply chain interruptions, adverse effects on healthcare systems and disruption of the global economy; our dependence on the success of our CAB technology platform; our ability to enroll patients in our ongoing and future clinical trials; the success of our current and future collaborations with third parties; our reliance on third parties for the manufacture and supply of our product candidates for clinical trials; our reliance on third parties to conduct our clinical trials and some aspects of our research and preclinical testing; and those other risks and uncertainties described in the section titled "Risk Factors" in our Annual Report on Form 10-K filed
with the Securities and Exchange Commission (SEC) on February 28, 2022 and in our Quarterly Reports on Form 10-Q filed with the SEC on May 4, 2022 and August 9, 2022 and our other reports as filed with the SEC. Forward-looking statements contained in this press release are made as of this date, and BioAtla undertakes no duty to update such information except as required under applicable law.
Internal Contact:
Richard Waldron
Chief Financial Officer
BioAtla, Inc.
rwaldron@bioatla.com
858.356.8945
External Contact: Bruce Mackle
LifeSci Advisors, LLC
bmackle@lifesciadvisors.com
BioAtla, Inc.